
You are now leaving www.klisyrihcp.com
You are now leaving KlisyriHCP.com and will be directed to an independent, third-party website. The Privacy Policy and the Terms of Use of the destination website will apply.
Quick Links
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
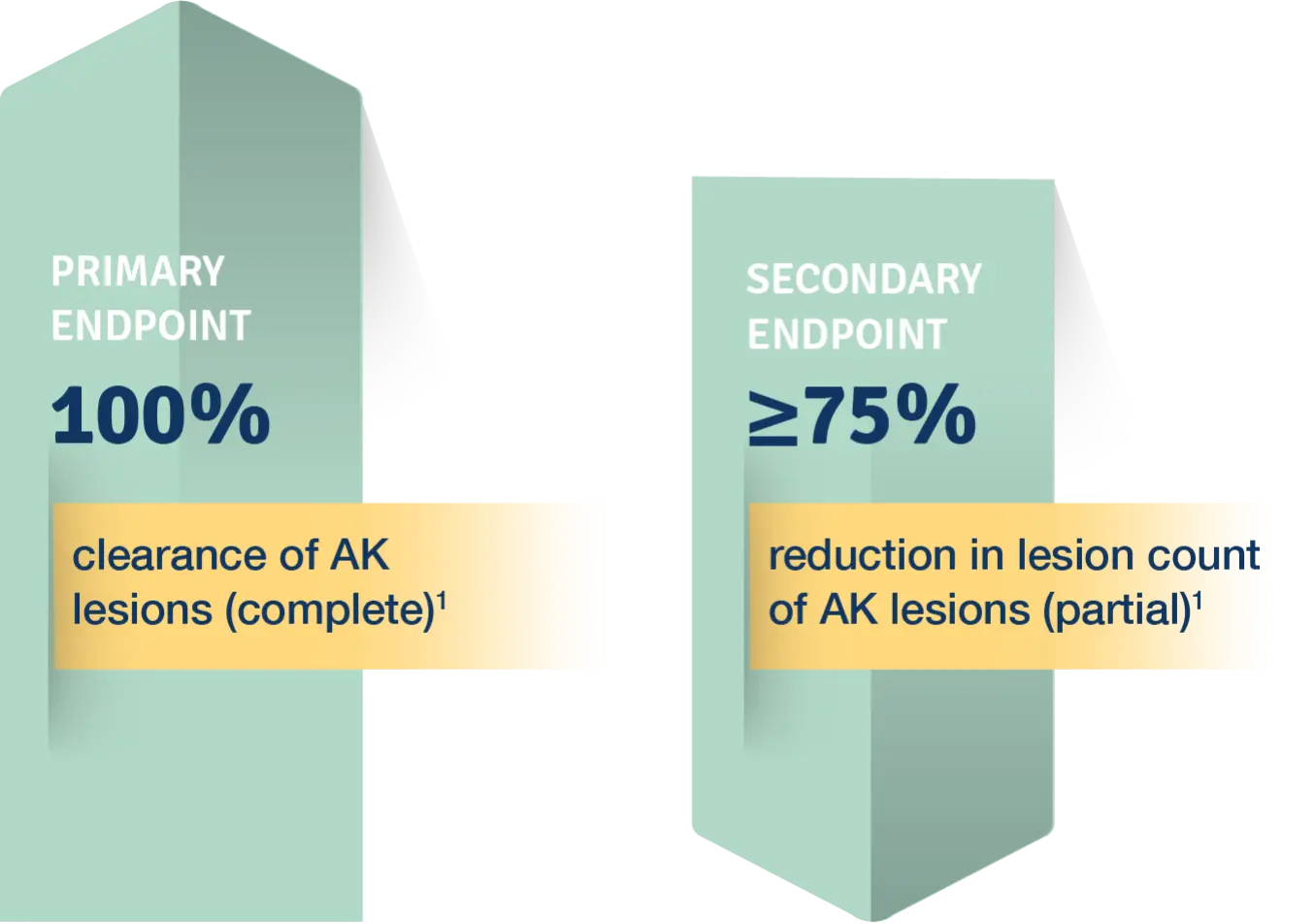
Primary Endpoint
100% AK clearance rates for the two Phase 3 studies for all subjects was 44% (n/N=77/175) KLISYRI® vs 5% (n/N=8/176) vehicle in study 1, and 54% (n/N=97/178) KLISYRI® vs 13% (n/N=22/73) vehicle in study 21†
*
Calculation based on pooled analysis of 702 patients from two Phase 3 studies evaluating all treatment locations (face or scalp): 49% KLISYRI® patients vs 9% vehicle.2
STUDY 1
STUDY 1 (% OF PATIENTS WITH complete
CLEARANCE ON DAY 57)1†

Actual clinical trial subject. Results may vary.
STUDY 2
STUDY 2 (% OF PATIENTS WITH complete
CLEARANCE ON DAY 57)1†
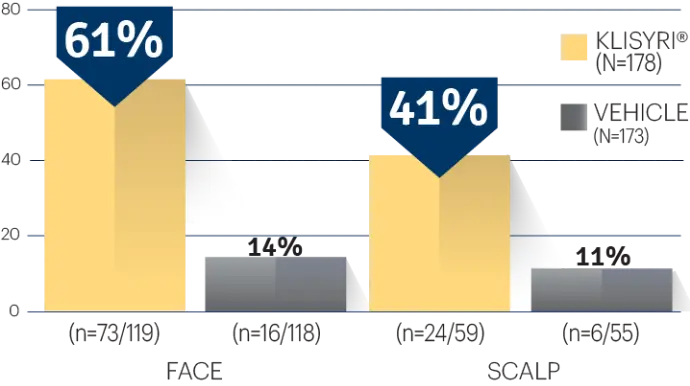
†P<0.0001 vs vehicle.3,4

Actual clinical trial subject. Results may vary.
Secondary Endpoint
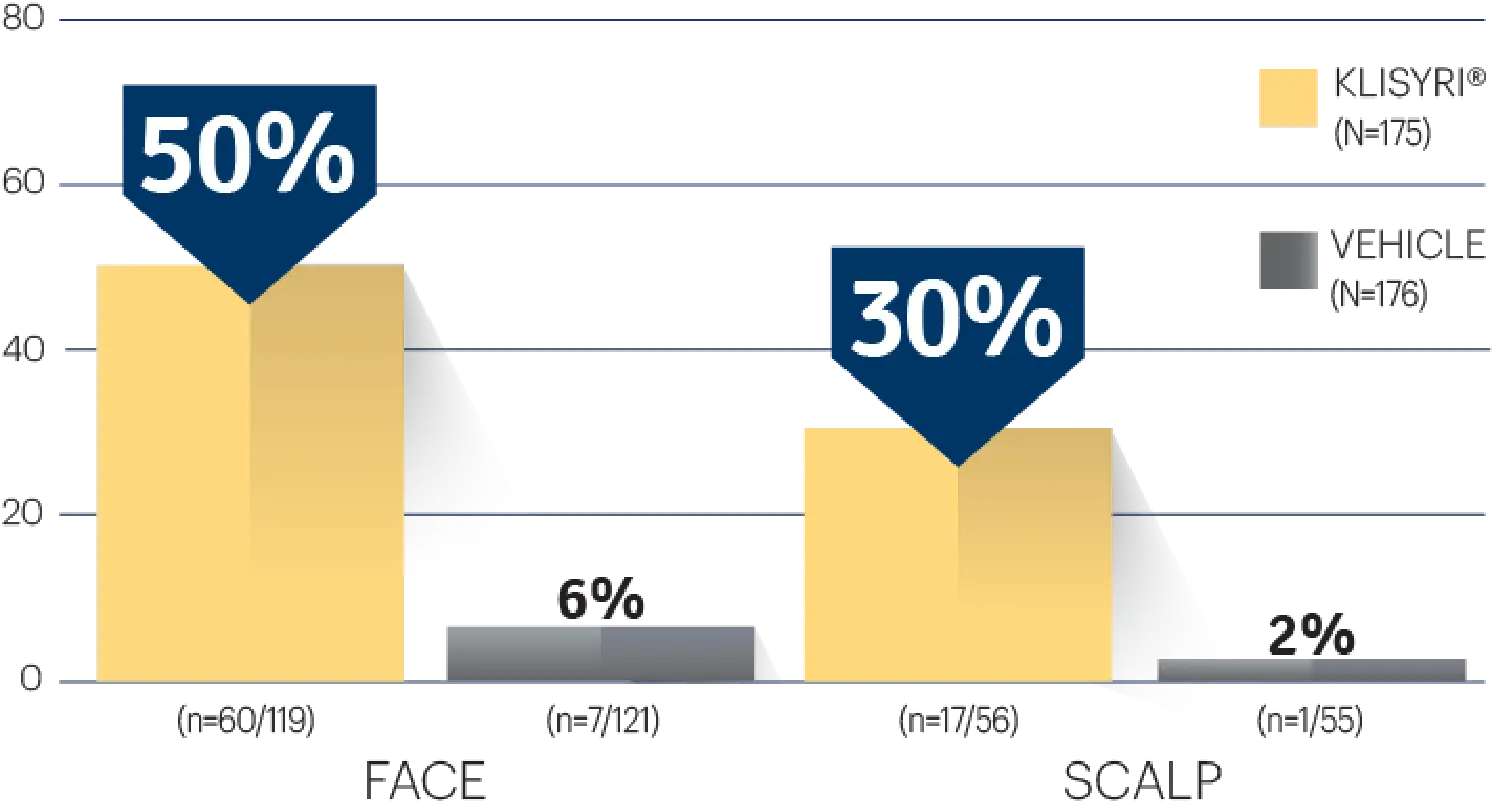
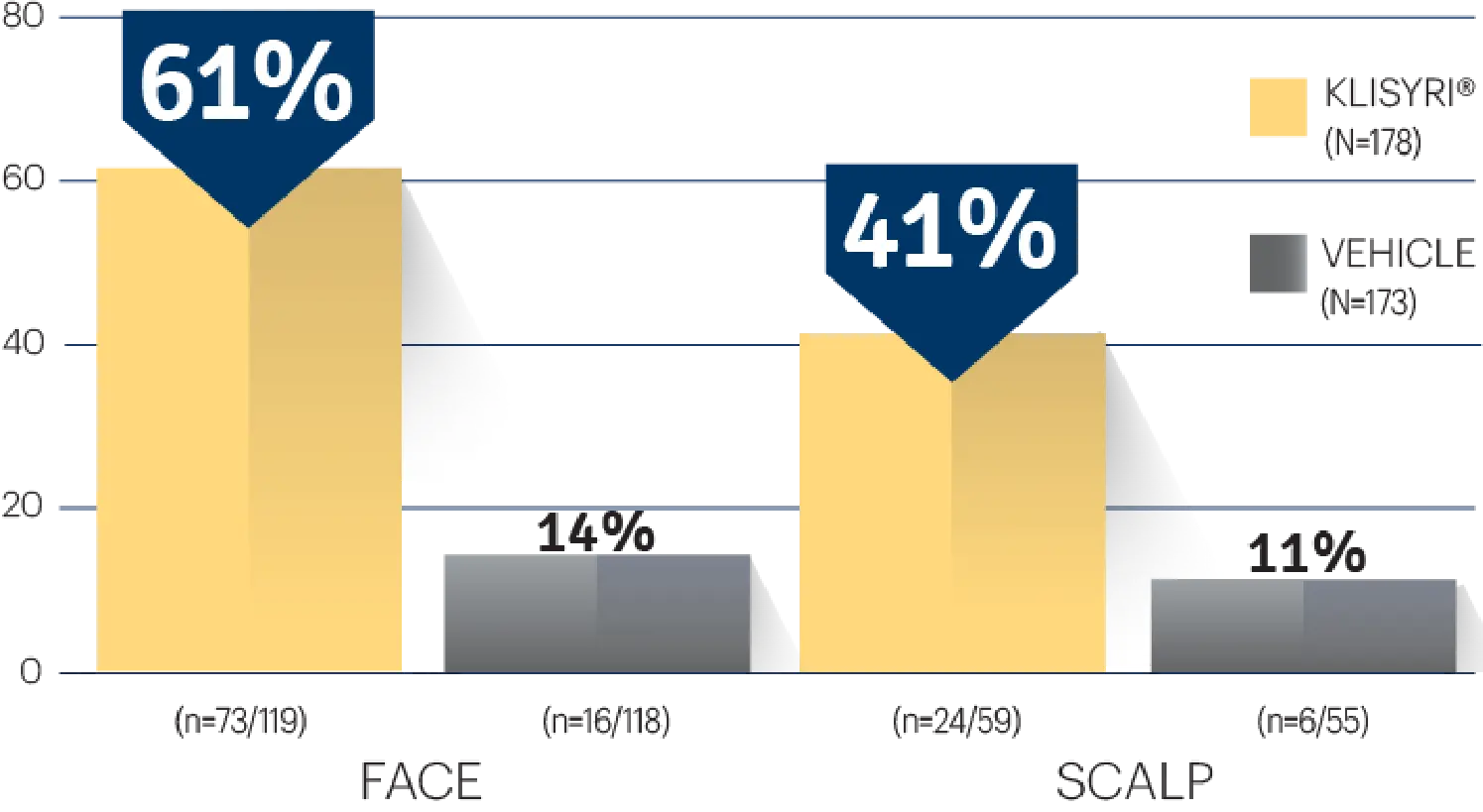
STUDY 1
STUDY 1 (% OF PATIENTS WITH ≥75%
CLEARANCE ON DAY 57)1‡


Actual clinical trial subject. Results may vary.
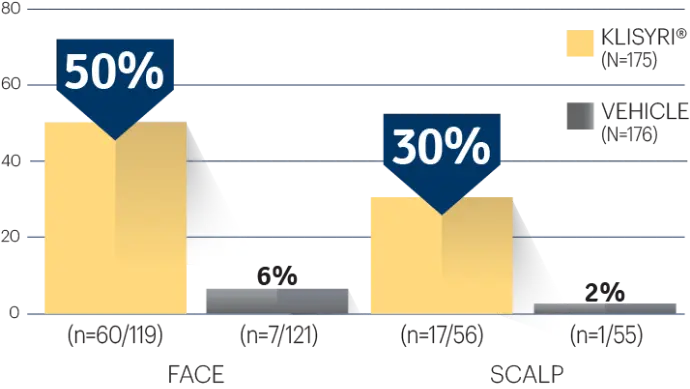
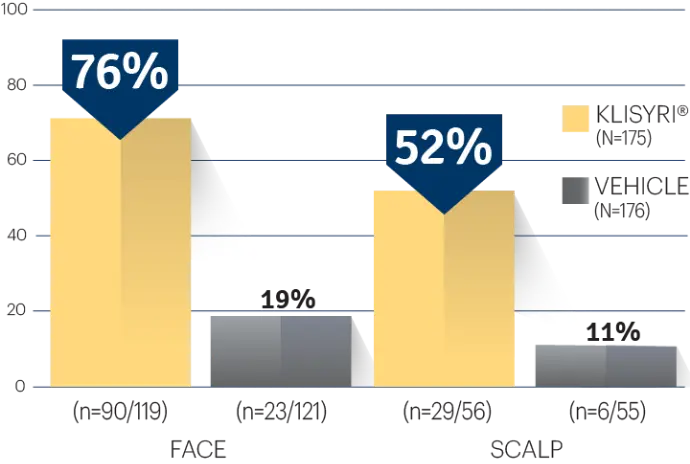
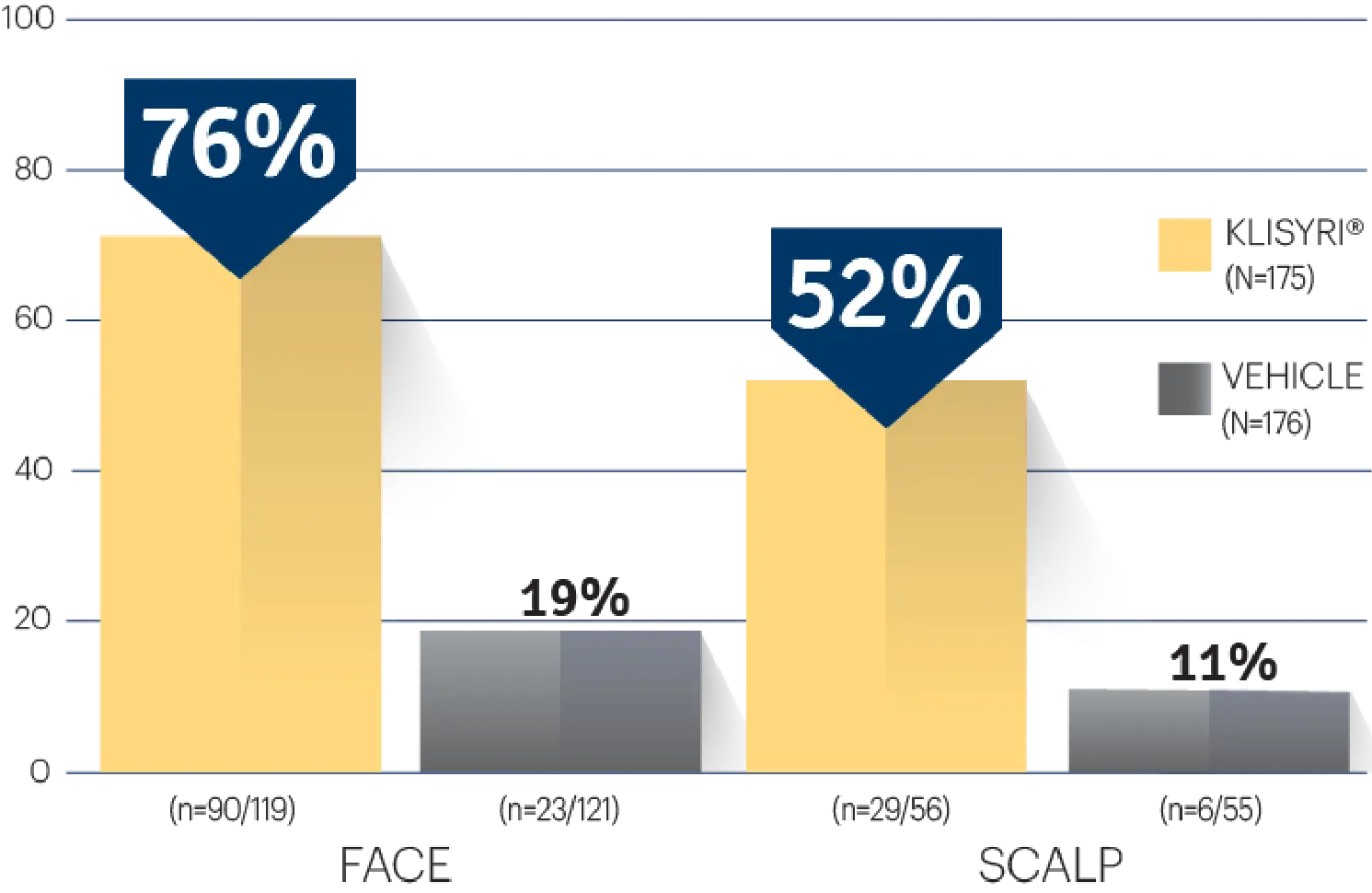
STUDY 2
STUDY 2 (% OF PATIENTS WITH ≥75%
CLEARANCE ON DAY 57)1‡
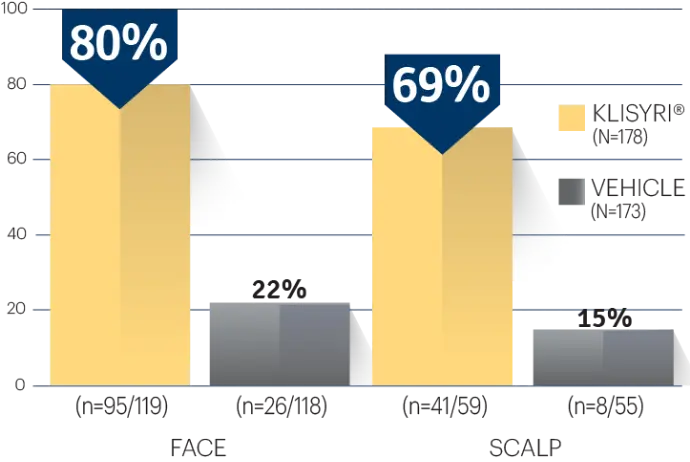
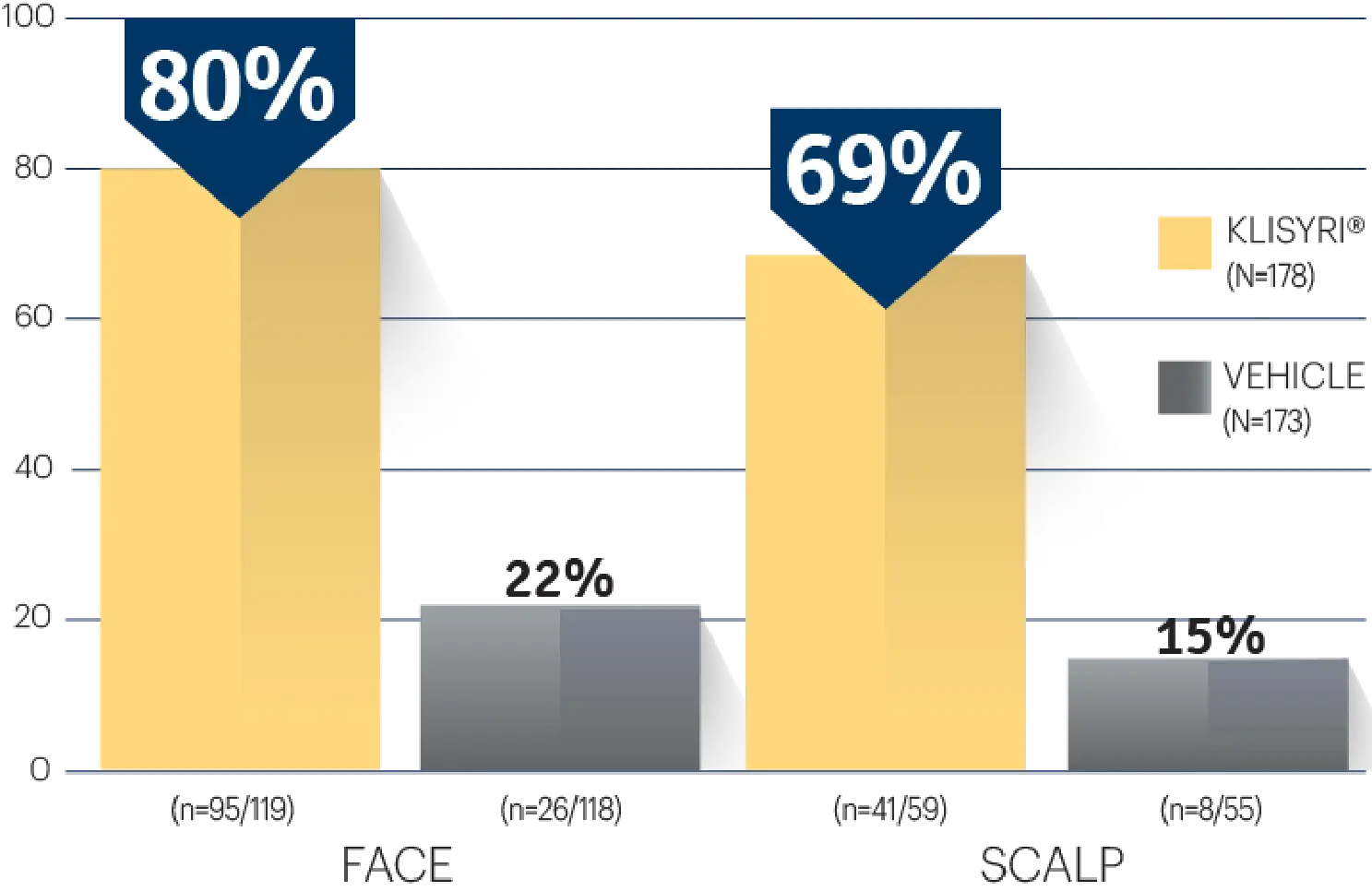
‡P<0.0001 vs vehicle.3,4

Actual clinical trial subject. Results may vary.
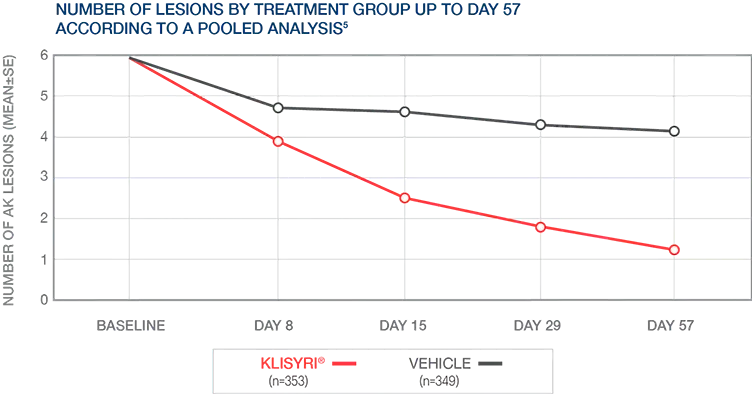
Reduction in AK lesion count to Day 57 was significantly greater (P<0.0001) than vehicle for all postbaseline visits until day 575
STUDY DESIGN

Identical, double-blind, randomized, vehicle-controlled clinical trials—1:1 randomization1
Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp1

Clinically typical, visible, and discrete AK lesions in a contiguous 25 cm2area1§
STUDY ENDPOINTS
Subjects received 5 consecutive days of once-daily treatment with either KLISYRI® (n=353) or vehicle control (n=349) to the treatment field1
§
25 cm2=3.92 inches.
AK: actinic keratosis.
SE: standard
error.
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp.
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment. Occlusion after topical application of KLISYRI is more likely to result in irritation.
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
To report an adverse event or product complaint, call or email: Medical Affairs and Customer Relations • Phone: 1-866-665-2782 • Fax: 510-595-8183 • Email: almirallmc@eversana.com
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp.
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment. Occlusion after topical application of KLISYRI is more likely to result in irritation.
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
To report an adverse event or product complaint, call or email: Medical Affairs and Customer Relations • Phone: 1-866-665-2782 • Fax: 510-595-8183 • Email: almirallmc@eversana.com
References:
1. KLISYRI® [package insert]. Malvern, PA: Almirall, LLC, 2021. 2. Data on file. 2021. Pooled Data Analysis. 3. Data on file. 2019. KX01-AK-004. 4. Data on file. 2019. KX01-AK-003. 5. Blauvelt A, Kempers S, Schlesinger T, et al. Tirbanibulin ointment 1% for actinic keratosis (AK): pooled data from two phase 3 studies. Presented at: 40th Annual Fall Clinical Dermatology Conference (Fall CDC 2020); Virtual Congress; October 29-November 1, 2020. 6. Data on file. 2021. Pivotal Clinical Trials.